
Chapter 7
Gradient Echo Imaging Methods
 |
Chapter 7 |
| Link to Book Table of Contents | Chapter Contents Shown Below |
It is
possible to produce an echo event by applying a magnetic field gradient without
a 180˚ RF pulse to the tissue as in the spin echo methods. There are several
imaging methods that use the gradient echo technique to produce the RF signals
and these make up the gradient echo family of methods.
The primary advantage of the gradient echo methods over the spin echo
methods is that gradient echo methods perform faster image acquisitions.
Gradient echo methods are generally considered to be among the faster imaging
methods. They are also used in some of the angiographic applications because
gradient echo generally produces bright blood, as we will see in Chapter 12, as
well as for functional imaging, as described in Chapter 13. One limitation of
the gradient echo methods is they do not produce good T2-weighted images, as
will be described later in this chapter. However, by combining the gradient and
spin echo methods, this limitation can be overcome.
At this time we will develop the concept of gradient echo and then
consider the specific gradient echo imaging methods and their characteristics.
Transverse magnetization is present only when a sufficient quantity of protons
are spinning in-phase in the transverse plane. As we have seen, the decay
(relaxation) of transverse magnetization is the result of proton dephasing. We
also recall that an RF signal is being produced any time there is transverse
magnetization and the intensity of the signal is proportional to the level of
magnetization.
With the spin echo technique we use an RF pulse to rephase the protons after they have been dephased by inherent magnetic field inhomogeneities and susceptibility effects within the tissue voxel. With the gradient echo technique the protons are first dephased, on purpose, by turning on a gradient and then rephased by reversing the direction of the gradient, as shown in Figure 7-1.
|
Figure Figure 7-1. The gradient echo process using a magnetic field
gradient to produce an echo event during the FID. |
|
A
gradient echo can only be created when transverse magnetization is present. This
can be either during the free induction decay (FID) period or during a spin echo
event. In Figure 7-1 the gradient echo is being created during the FID. Let us
now consider the process in more detail.
First, transverse magnetization is produced by the excitation pulse. It
immediately begins to decay (the FID process) because of the magnetic field
inhomogeneities within each individual voxel. The rate of decay is related to
the value of T2*. A short time after the excitation pulse a gradient is applied,
which produces a very rapid dephasing of the protons and reduction in the
transverse magnetization. This occurs because a gradient is a forced
inhomogeneity in the magnetic field. The next step is to reverse the direction
of the applied gradient. Even though this is still an inhomogeneity in the
magnetic field, it is in the opposite direction. This then causes the protons to
rephase and produce an echo event. As the protons rephase, the transverse
magnetization will reappear and rise to a value determined by the FID process.
The gradient echo event is a rather well-defined peak in the transverse
magnetization and this, in turn, produces a discrete RF signal.
The TE is determined by adjusting the time interval between the
excitation pulse and the gradients that produce the echo event. TE values for
gradient echo are typically much shorter than for spin echo, especially when the
gradient echo is produced during the FID.
Small Angle Gradient Echo Methods
The
gradient echo technique is generally used in combination with an RF excitation
pulse that has a small flip angle of less than 90˚. We will discover that the
advantage of this is that it permits the use of shorter TR values and this, in
turn, produces faster image acquisition.
One source of confusion is that each manufacturer of MRI equipment has
given his gradient echo imaging methods different trade names. In this text we
will use the generic name of small angle gradient echo (SAGE) method.
The SAGE method generally requires a shorter acquisition time than the
spin echo methods. It is also a more complex method with respect to adjusting
contrast sensitivity because the flip angle of the excitation pulse becomes one
of the adjustable protocol imaging factors.
Excitation/Saturation-Pulse
Flip Angle
We recall that the purpose of the excitation/ saturation pulse applied at the beginning of an imaging cycle is to convert or flip longitudinal magnetization into transverse magnetization. When a 90° pulse is used, all of the existing longitudinal magnetization is converted into transverse magnetization, as we have seen with the spin echo methods. The 90° pulse reduces the longitudinal magnetization to zero (i.e., complete saturation) at the beginning of each imaging cycle. This then means that a relatively long TR interval must be used to allow the longitudinal magnetization to recover to a useful value. The time required for the longitudinal magnetization to relax or to recover is one of the major factors in determining acquisition time. The effect of reducing TR when 90° pulses are used is shown in Figure 7-2.
|
7-2. The effect of reducing TR on the recovery of longitudinal
magnetization within a cycle and the resulting signal intensity when
using 90° pulses. |
|
As
the TR value is decreased, the longitudinal magnetization grows to a lower value
and the amount of transverse magnetization and RF signal intensity produced by
each pulse is decreased. The reduced signal intensity results in an increase in
image noise as described in Chapter 10. Also, the use of short TR intervals with
a 90° pulse (as in spin echo) cannot produce good PD or T2-weighted images.
One approach to reducing TR and increasing acquisition speed without
incurring the disadvantages that have just been described is to use a pulse that
has a flip angle of less than 90°. A small flip-angle (<90°) pulse converts only
a fraction of the longitudinal magnetization into transverse magnetization. This
means that the longitudinal magnetization is not completely destroyed or reduced
to zero (saturated) by the pulse, as shown in Figure 7-3.
|
Figure 7-3. The effect of using small flip angle pulses on longitudinal magnetization. |
|
Reducing the flip angle has two effects that must be considered together. The effect that we have just observed is that the longitudinal magnetization is not completely destroyed and remains at a relatively high level from cycle to cycle, even for short TR intervals. This will increase RF signal intensity compared to the use of 90° pulses. However, as the flip angle is reduced, a smaller fraction of the longitudinal magnetization is converted into transverse magnetization. This has the effect of reducing signal intensity. The result is a combination of these two effects. This is illustrated in Figure 7-4.
|
Figure 7-4. The effect of pulse flip angle on the level of both
longitudinal and transverse magnetization after the pulse is applied. |
|
Here we see that as the flip angle is increased over the range from 0–90°, the level of longitudinal magnetization at the beginning of a cycle decreases. On the other hand, as the angle is increased, the fraction of this longitudinal magnetization that is converted into transverse magnetization increases and RF signal intensity increases. The combination of these two effects is shown in Figure 7-5.
|
Figure 7-5. The relationship of signal intensity to flip angle. |
|
Here we
see how changing flip angle affects signal intensity. The exact shape of this
curve depends on the specific T1 value of the tissue and the TR interval. For
each T1/TR combination there is a different curve and a specific flip angle that
produces maximum signal intensity.
Let us now use Figure 7-6 to compare the magnetization of two tissues with different T1 values as we change flip angle.
|
Figure 7-6. The effect of flip angle on contrast. |
|
Contrast
between the two tissues is represented by the difference in magnetization
levels. At this point we are assuming a short TE and considering the contrast
associated with only the longitudinal magnetization. The flip-angle range is
divided into several specific segments as shown.
With the
SAGE method the contrast sensitivity for a specific tissue characteristic is
controlled by three protocol factors. As with spin echo, TR and TE have an
effect. However, the flip angle becomes the factor with the greatest effect on
contrast. We will now see how changing flip angle can be used to select specific
types of contrast with a basic gradient echo method.
Relatively large flip angles (45°–90°) produce T1 contrast. This is what we
would expect because large flip angles (close to 90°) and short TR and TE values
are similar to the factors used to produce T1 contrast with the spin echo
method. Here, with gradient echo, we observe a loss of T1 contrast as the flip
angle is decreased significantly from 90°.
There is
an intermediate range of flip-angle values that produces very little, if any,
contrast. This is the region in which the PD and T1 contrast cancel each other
for many tissues, such as gray and white matter.
Relatively low flip-angle values produce PD contrast. As the flip angle is
reduced within this region, there is a significant decrease in magnetization and
the resulting signal intensity.
Up to
this point we have observed generally how changing the flip angle of the
excitation pulse affects signal intensity and contrast. In the SAGE imaging
method the flip angle is one of the imaging factors that must be adjusted by the
user. However, it becomes somewhat complex because the specific effect of flip
angle is modified by the other imaging factors and techniques used to enhance a
specific type of contrast.
We
recall that T2 contrast is produced by the decay characteristics of transverse
magnetization and that there are two different decay rates, T2 and T2*. The
slower decay rate is determined by the T2 characteristics of the tissue. The
faster decay is produced by small inhomogeneities within the magnetic field
often related to variations in tissue susceptibility differences. This decay
rate is determined by the T2* of the tissue environment. When a spin echo
technique is used, the spinning protons are rephased, and the T2* effect is
essentially eliminated. However, when a spin echo technique is not used, the
transverse magnetization depends on the T2* characteristics. The gradient echo
technique does not compensate for the inhomogeneity and susceptibility effect
dephasing as the spin echo technique does. Also, without using a spin echo
process the long TE values necessary to produce T2 contrast cannot be achieved.
Therefore, a basic gradient echo imaging method is not capable of producing true
T2 contrast. The contrast will be determined primarily by the T2*
characteristics. The amount of T2* contrast in an image is determined by the
selected TE value. In general, longer TE values (but short compared to those
used in spin echo) produce more T2* contrast.
In
addition to using combinations of TR, TE, and flip angle to control the contrast
characteristics, some gradient echo methods have other features for enhancing
certain types of contrast.
When SAGE methods are used with relatively short TR values, there is the
possibility that some of the transverse magnetization created in one imaging
cycle will carry over into the next cycle. This happens when the TR values are
in the same general range as the T2 values of the tissue. SAGE methods differ in
how they use the carry-over transverse magnetization.
A typical SAGE sequence is limited to one RF pulse per cycle. If
additional pulses were used, as in the spin echo techniques, they would affect
the longitudinal magnetization and upset its condition of equilibrium. However,
because of the relatively short TR values it is possible for the repeating
small-angle excitation pulses to produce a spin echo effect. This can occur only
when the TR interval is not much longer than the T2 value of the tissue.
Associated with each excitation pulse, there are actually two components
of the transverse magnetization. There is the FID produced by the immediate
pulse and a spin echo component produced by the preceding pulses. The spin echo
component is related to the T1 characteristics of the tissue. The FID component
is related to the T2* characteristics. The contrast characteristics of the
imaging method are determined by how these two components are combined.
Different combinations are obtained by altering the location of the gradient
echo event relative to the transverse magnetization and by turning the spin echo
component on or off as described below.
When
both the FID and spin echo components are used, an image with mixed contrast
characteristics will be obtained. This method produces a relatively high signal
intensity compared to the methods described below.
Spoiling and T1 Contrast
Enhancement
An image
with increased Tl contrast is obtained by suppressing the spin echo component.
This is known as spoiling. The spin echo component, which is a carryover
of transverse magnetization from previous cycles, can be destroyed or spoiled by
either altering the phase relationship of the RF pulses or by applying gradient
pulses to dephase the spinning protons.
The basic SAGE method discussed up to this point permits faster (than
spin echo) image acquisition because the TR can be set to shorter values.
However, the gradient echo process can be used in methods that provide fast
acquisition based on an entirely different principle. We will now consider
methods that achieve their speed by filling many rows of k space during one
acquisition cycle.
In Chapter 5 we saw that in the acquisition phase the signal data is
being directed into k space from which the image will be reconstructed. The k
space is filled one row at a time. The number of rows in the k space for a
specific image depends on the required image detail. The process that directs
the signals into a specific row of k space is the spatial encoding function
performed by one of the gradients. This will be described in Chapter 9. In
conventional spin echo and SAGE imaging only one row of k space is filled with
each imaging cycle. This is because there is only one echo signal produced per
cycle that can be encoded to go to a specific row of k space. This means that
the size of k space determines the minimum number of cycles that an acquisition
must have. We are about to see some gradient echo methods that can fill many
rows of k space in one imaging cycle. This is achieved by using the gradient
echo process to produce many echo events from the transverse magnetization that
is present during one cycle.
Echo Planar Imaging (EPI) Method
Echo
planar is the fast gradient echo imaging method that is capable of acquiring a
complete image in a very short time. However, it requires an MRI system equipped
with strong gradients that can be turned on and off very rapidly. All systems do
not have this capability. The EPI method consists of rapid, multiple gradient
echo acquisitions executed during a single spin echo event. The unique
characteristic of this method is that each gradient echo signal receives a
different spatial encoding and is directed into a different row of k space. The
actual spatial encoding process will be described in Chapter 9. Here we are
considering only the general concept of EPI and how it achieves rapid
acquisition.
The basic EPI method is illustrated in Figure 7-7.
|
Figure 7-7. The production of many gradient echo events within one
imaging cycle with the echo planar imaging (EPI) method. |
|
Here
we see the actions that occur within one imaging cycle. The cycle usually begins
with a spin echo pulse sequence that produces a spin echo event consisting of a
period of transverse magnetization as described in Chapter 6. In conventional
spin echo imaging we obtain only one signal (and fill one row of k space) from
this period of magnetization. What we are about to do with EPI is to chop this
one spin echo event into many shorter gradient echo events. The signals from
each gradient echo event will receive different spatial encodings and fill
different rows of k space.
It is possible to fill all of the k space and acquire a complete image in
one cycle. This is described as single shot EPI. This is not always
practical because it might place some limitations on the image quality that can
be achieved and is also very demanding on the gradients. A more practical
approach is to divide the acquisition into multiple shots (cycles) with each
filling some fraction of the total k space.
The important factor is the number of gradient echo events created in
each cycle. This is an adjustable protocol factor and is generally known as the
EPI speed factor. This is the factor by which the acquisition time is
reduced compared to a conventional method using the same TR value.
Gradient and Spin Echo (GRASE) Method
The
GRASE method is, as the name indicates, a combination of gradient and spin echo
methods. It provides the fast acquisition capability of gradient echo (EPI) with
the superior contrast characteristics of spin echo, including the ability to
produce T2 images.
The GRASE method is illustrated in Figure 7-8 where we see the actions occurring within one imaging cycle.
|
Figure 7-8. The use of the GRASE method to fill many rows of k space and
produce a fast acquisition. |
|
The
basic cycle is a multiple spin echo as described in Chapter 5. The difference is
that in conventional multiple echo, each of the echo events have different TE
values and are used to form several images; typically, a PD and a T2 image with
the same acquisition. Here the multiple spin echo is used for a different
purpose. The multiple spin echoes are used to cover more of k space. As we see,
each of the spin echo events is cut into many gradient echoes by the EPI
process. This reduces the acquisition time by two factors: the total speed
factor is the number of multiple spin echoes multiplied by the EPI speed factor.
Both
SAGE with short TR values and EPI can produce very rapid acquisitions. However,
the short time intervals between the gradient echo events do not provide
sufficient time for good longitudinal magnetization contrast (T1 or PD) to be
formed. This problem is solved by “preparing” the magnetization and forming the
contrast just one time at the beginning of the acquisition cycle, as shown in
Figure 7-9. Two options are shown.
|
Figure 7-9. Using preparation pulses to produce longitudinal
magnetization contrast prior to a rapid gradient echo acquisition. |
|
The longitudinal magnetization is prepared by applying either a
saturation pulse, as in the the inversion-recovery method or an inversion pulse,
as in the inversion-recovery method. As the longitudinal magnetization relaxes,
contrast is formed between tissues with different T1 and PD values. After a time
interval [TI or TS (Time after Saturation)] selected by the operator, a rapid
gradient echo acquisition begins.
The total acquisition time for this method l-is the time required by the
acquisition cycles plus the TI or TS time interval.
Mind Map Summary
Gradient Echo Imaging Methods
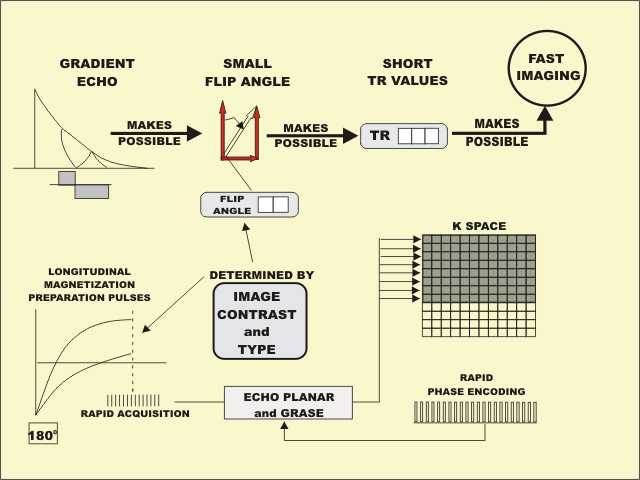
The common characteristic of the gradient echo imaging methods is that a
magnetic field gradient is used to produce the echo event rather than a 180° RF
pulse, as is used in the spin echo methods. One of the principal advantages of
the gradient echo process is that it is a relatively fast imaging method.
By using a gradient, and not an RF pulse, to produce the echo event, it
is possible to use saturation/excitation pulses with flip angles less than 90˚;
thereby all the longitudinal magnetization is not destroyed (saturated) at the
beginning of each cycle. Because some longitudinal magnetization carries over
from cycle to cycle, it is possible to reduce the TR value and still produce
useful signal levels. The reduced TR values result in faster imaging. The flip
angle of the RF pulse is an adjustable protocol factor that controls the type of
contrast produced.
Echo planar imaging is a gradient echo method in which many echo events,
each with a different phase encoding step, are created during each imaging
cycle. This makes it possible to fill multiple rows of k space, which results in
very fast imaging. GRASE is an imaging method that combines the principles of
echo planar and fast (turbo) spin echo to produce rapid imaging acquisitions.
When very fast gradient echo methods are used, there is not sufficient
time between the echo events for significant tissue relaxation and contrast to
develop. Therefore, the desired contrast is developed at the beginning of the
acquisition by applying either inversion or saturation “magnetization
preparation” pulses. Then, when the desired contrast has developed, a rapid
acquisition is performed.